Login to your facilities instance of REDCap. If you are using HMRL’s instance, this can be accessed through https://fhs.cac.queensu.ca/HMRL/
To create an account under the HMRL instance email jereme.outerleys@queensu.ca
Download the “Multi-Centre-Intake-Template” and “Multi-Centre-Visit-Data-Template” found HERE
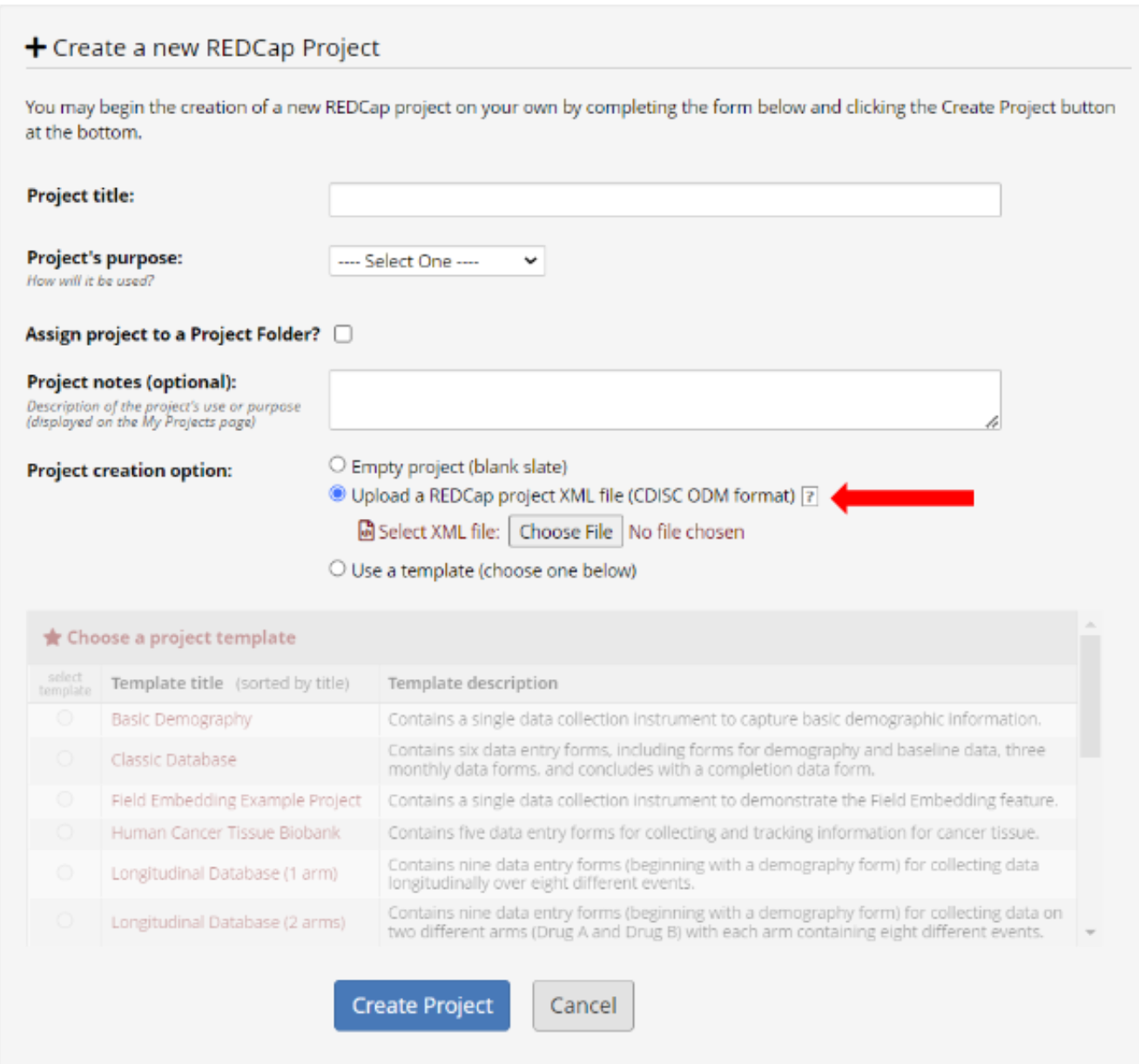
In REDCap click “New Project” found on the top bar.
Click 'Create Project.
Repeat steps 3-5 for the 'Multi-Centre-Visit-Data-Template' .xml file that was downloaded in step 2.
Locate the participant in the Intake form using their name, or other identifying information.
Once you have confirmed you have the right participant, locate their Project Record ID Number (EPS) found near the bottom of their consent form.
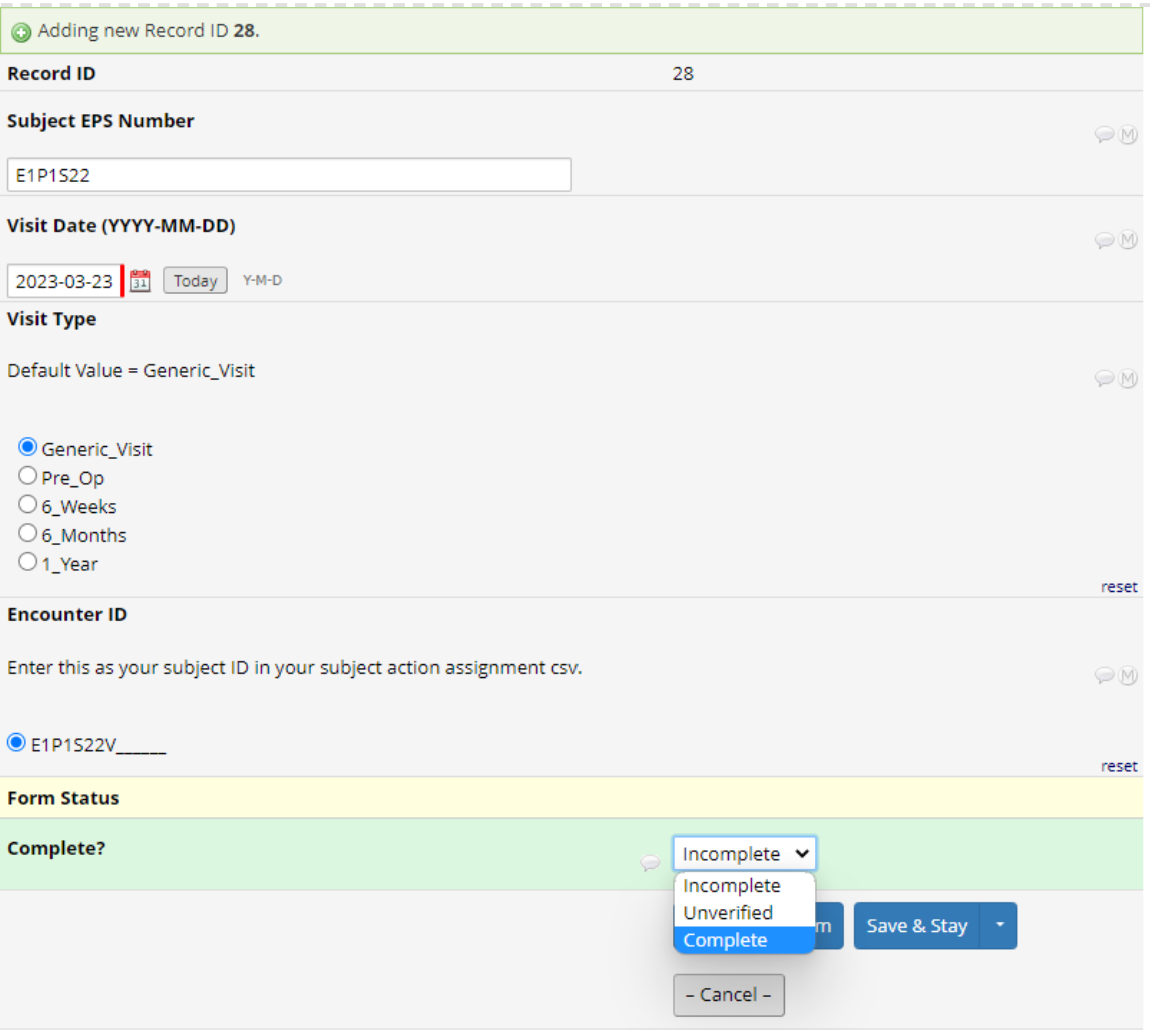
Go to the 'Multi-Centre-Visit-Data' Form and click the 'Add new record' button. This will create a new empty record.
Open the 'Visit info' Instrument and input the subject's ESP number found in step 2.
Specify the visit type (note that the default value is 'Generic Visit').
Complete the form, then save and exit the form.
Note: Don't worry that the Encounter ID appears to have blanks, it will autofill once the form is saved and completed.
There are two separate REDCap projects, an intake project and a data visit project. The intake contains personal, identifiable information about the subject whereas the data visit project contains only de-identified information pertaining to the data collection itself.
Summary: In this instrument, the Participant is screened for eligibility and their ESP number is created (a project ID that is unique to the subject)
Participant Input: All fields
Researcher Input: N/A (past initial setup)
Setup: As part of the initial setup, you will need to change the default values of the ESP number. The lead institution will provide the default numbers for you.
Summary: This is the consent form that the participant will sign. Everything contained on this page will be saved within the repository. The final consent pdf is saved once the participant has completed this form AND one of the study staff or the PI has signed the study staff form certifying that all questions the participant may have had were answered, and verified that the consent has been completed. Completed consent PDFs can be found under 'File repository' located under the 'Applications' tab in REDCap’s side menu bar.
Subject Input: All Fields
Researcher Input: N/A
Setup: Add your institution's consent form (by pasting it in the indicated field) into this instrument, the current setup is a suggestion and how the HMRL’s is currently formatted, but change it to best fit your consent requirements.
Summary: This instrument is where the participant will put in their contact information, preferred name and pronouns.
Participant Input: All fields
Researcher Input: N/A
Setup: N/A
Summary: Participant's Date of birth. There is an internal validation that hides the submit button until real dates have been inputted. To use this validation, the Hide Submit External Module must be activated.
Participant Input: All fields
Researcher Input: N/A
Setup: Enable Hide Submit External Module
Summary: Asks participants demographics questions (ethnicity, gender etc.). The ethnicity options currently match the Canadian consensus. The age of the participant is auto-calculated in this instrument but hidden from the participant.
Participant Input: All fields
Researcher Input: N/A
Setup: N/A
Summary: This instrument is where the consenting study staff certifies that all questions the participant may have had were answered, and verify that the consent has been completed. Once this form is complete, it will get merged with the participant’s consent PDF using the Multi-signature External Module.
Participant Input: N/A
Researcher Input: All fields
Setup: Enable Multi-signature Module
Summary: This is a completely optional instrument. It is used by the radiologists to provide a KL Score.
Participant Input: N/A
Researcher Input: N/A
Setup: N/A
Summary: This instrument is used to generate the subject’s ID and add some additional information about the visit.
When a participant is filling in REDCap through survey mode, all fields will be auto-filled (either auto-generated or with the default value)
If a new record is being created by the researcher, the Subject’s EPS Number and visit type will need to be manually filled in. The EPS number is a unique identifier that is created in the intake form after the consent has been completed.
Subject Input: N/A
Setup: N/A
Summary: Asks the participant which knee they are in for and screens for other musculoskeletal/neurological disorders (Both patients and controls fill this out)
Subject Input: All Fields
Researcher Input: N/A
Setup: N/A
Summary: Pre-approved REDCap Eq5d5l PROMS template (Completed by Subjects and controls)
Subject Input: All Fields
Researcher Input: N/A
Setup: N/A
Summary: PROMS completed by the patient only. The sde (right/left) is determined by which knee is affected (defined in Reason for Visit Instrument) and is queued accordingly.
Subject Input: All Fields
Researcher Input: N/A
Setup: N/A
Summary: PROMS completed by the patient only.
Subject Input: All Fields
Researcher Input: N/A
Setup: N/A
Summary: PROMS completed by the patient only. The side (right/left) is determined by which knee is affected (defined in Reason for Visit Instrument) and is queued accordingly.
Subject Input: All Fields
Researcher Input: N/A
Setup: N/A
Summary: auto generates a unique participant QR Code. Use this QR code if using the QR code data sheet method (see QR Code Data Sheet).
Subject Input: N/A
Researcher Input: Autogenerated if participant has completed survey, upon repeat visit, this form will need to be manually saved to present QR code.
Setup: N/A
Summary: Asks about the patient's surgical history and details, such as the name of the surgeon, case type, side of the body performed on, and patient diagnosis.
Subject Input: N/A
Researcher Input: manually inputted by researchers after data collection based on information provided by patient and physiotherapist.
Setup: N/A
Summary: Record patient information on the date of surgery and clinical research nurse, if applicable.
Subject Input: N/A
Researcher Input: N/A
Setup: N/A
Summary: Asks for patient's clinical status of affected knee, history of any joint replacements, and analgesic medications taken regularly and on the dat of data collection. Physiotherapist comments on the knee, the name of the referred surgeon, and additional study staff comments are also recorded within this section. The date of the surgery, if known, should be recorded.
Subject Input: N/A
Researcher Input: N/A
Setup: N/A
Summary: Overview of data collection process and trials. FIl out details regarding the set-up, date of visit, calibration, pariticpant completed conset intake, clothing and potential flogs, mobility aids, and trial information. Trial overview includes:
Timed up and Go (TUG)
Quiet Stand
Squat (SLR and SLL)
Tandem Stand
FoamEC
CircuitOrtho (Sit to Stand, Stairs)
Walk
Fast
Include Slow Walk if participant is a control. Any reasoning for exclusion or inclusion of mobility aids within each trial should be recorded within reasoning notes and researcher notes.
Subject Input: N/A
Researcher Input: N/A
Setup: N/A